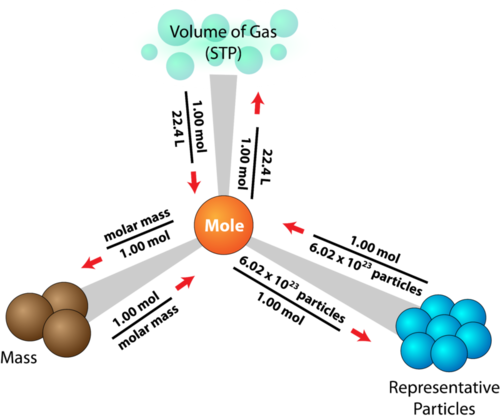
The mole is a standard unit of measurement in chemistry. It is defined as 6.02 * 1023 Avogadro's number) atoms or molecules of a substance. Along with molar mass, these values can be used as conversion factors to find the amount of particles or the mass of a sample. This road map explains all the conversions
Helpful Links:
http://chemwiki.ucdavis.edu/Physical_Chemistry/Atomic_Theory/The_Mole_and_Avogadro's_Constant
No comments:
Post a Comment