The driving force of an acid-base reaction is the production of water.This reaction also produces a salt. When getting a net ionic equation, weak acids and bases won't dissociate while strong ones will. To determine strong acids and bases, remember that strong acids have oxygens that outnumber the hydrogens by two or more while strong bases have group 1 and 2 metals with the hydroxide ion. The key to this unit is to memorize the rules so I will study a little every night and do the practice problems on schoology to prepare!
Helpful links
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/acidbase.html
Monday, November 23, 2015
Sunday, November 22, 2015
Solubility Lab
In the solubility lab, we combined many different reagents to see if a precipitate formed or not. Before the lab, we used our solubility rules to predict whether a precipitate will form or not for every reaction. Some reactions that involved hydroxide didn't follow my predictions. Overall, the lab was enjoyable and it helped me practice solubility rules.
Helpful links
Monday, November 16, 2015
Chemical Composition Test
Today we took the post test for the chemical composition unit. I would say that this was the hardest test yet in this class. I worked for the whole class period and had rework many questions to double check them. Many problems were about application and some had multiple questions to them. In the end, I guessed on the last few questions as I ran out of time. Since I will need to study this later on, here are some helpful links.
http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/section/10.9/
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://www.chemteam.info/Mole/EmpiricalFormula.html
http://www.chemteam.info/Mole/Emp-formula-given-mass-data.html
http://www.ck12.org/book/CK-12-Chemistry-Concepts-Intermediate/section/10.9/
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
http://www.chemteam.info/Mole/EmpiricalFormula.html
http://www.chemteam.info/Mole/Emp-formula-given-mass-data.html
Friday, November 13, 2015
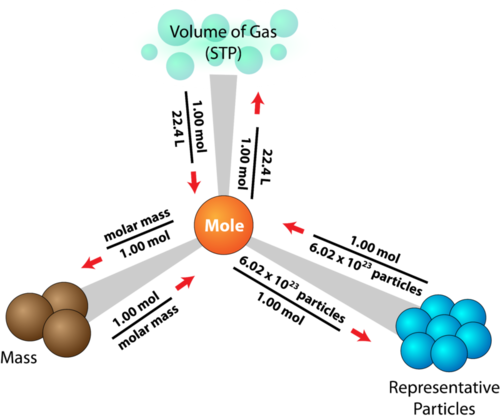
Mole Conversions
The mole is a standard unit of measurement in chemistry. It is defined as 6.02 * 1023 Avogadro's number) atoms or molecules of a substance. Along with molar mass, these values can be used as conversion factors to find the amount of particles or the mass of a sample. This road map explains all the conversions
Helpful Links:
http://chemwiki.ucdavis.edu/Physical_Chemistry/Atomic_Theory/The_Mole_and_Avogadro's_Constant
Formula of a Hydrate Lab
In this lab we determined the formula of a hydrate by heating the hydrate in a test tube. We measured the water driven off by heat and the anhydrous compound left to get the formula. Some groups didn't pass the pre-lab quiz so not all of the class got into the lab. Fortunately, me and my partner both got our questions right. I think this is the best lab we've done so far because we got to use the bunsen burner.
 |
| Heating the hydrate and driving off the water |
 |
| The end anhydrous compound with no water |
 |
| Measuring the compound |
Tuesday, November 3, 2015
Chemical Composition Pre Test
Yesterday we took the pre-test for this new unit. After I saw that the questions involved lots of math, I started guessing after attempting the first few questions. This unit will probably include lots of work. It will also require lots of math and I don't know if that will make this unit easier or harder. Chemical composition seems pretty hard so I hope I get through this unit!
| www.lohninger.com |
Subscribe to:
Comments (Atom)